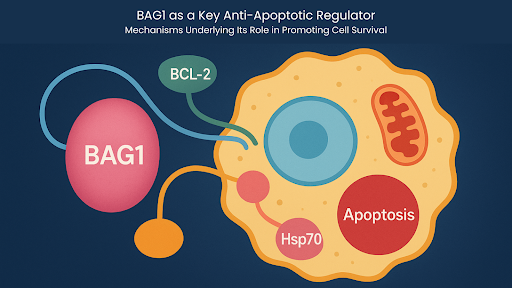
BAG1 (BCL-2–associated athanogene 1) is at the center of regulatory networks that protect cells from apoptosis. It interacts with the anti-apoptotic protein BCL-2, and it also operates at the intersection of chaperone activity, proteostasis, transcriptional regulation, and stress-response pathways for the survival of cells.
Its diverse interactions with BCL-2 family members, Hsp70/Hsc70 chaperones, nuclear hormone receptors, and components of the ubiquitin–proteasome system make BAG1 indispensable for cell survival under physiological and pathological conditions, including:
- Cancer
- Neurodegeneration
- Ischemic injury
BAG1 is a protein of high relevance, and researchers rely on tools such as the mouse BAG1 monoclonal antibody for the precise dissection of its biological roles.
BAG1 Strengthens BCL-2 Anti-Apoptotic Signaling
Its interaction with the BCL-2 family, particularly BCL-2 itself, is the defining characteristic of BAG1. The BAG1 domain is located in the C-terminal regions and binds to the BH4 domain of BCL-2 to stabilize the anti-apoptotic conformation of BCL-2.
This interaction enhances the ability of BCL-2 to suppress mitochondrial outer membrane permeabilization (MOMP). This prevents the release of cytochrome c and subsequent activation of caspase-9 and downstream effector caspases.
This mechanism is crucial during stress events such as:
- Oxidative damage
- Heat shock
- DNA damage
- Exposure to chemotherapeutic agents
Cells with elevated BAG1 expression are found to exhibit delayed mitochondrial depolarization and increased resistance to apoptotic triggers.
Studies using the mouse BAG1 monoclonal antibody help map the spatial distribution of BAG1 in mitochondrial stress models. These studies confirm BAG1’s co-localization with BCL-2-associated complexes during apoptosis inhibition.
Integration With Hsp70/Hsc70 Chaperone Machinery
In addition to its role in BCL-2 regulation, BAG1 also acts as an essential co-chaperone, functioning as a nucleotide exchange factor (NEF) for Hsp70 and Hsc70.
Hsp70 and Hsc70 are two major protein-folding chaperones that maintain proteome stability under stress. BAG1 promotes the release of ADP from Hsp70, thereby accelerating the chaperone cycle and enabling faster processing of client proteins.
Prevention of Protein Aggregation
Accumulation of misfolded proteins can overwhelm the cell under conditions such as heat shock, hypoxia, and oxidative stress. BAG1 helps Hsp70 resolve partially folded proteins. These unfolded proteins can activate apoptosis. The BAG1 interaction with Hsp70 reduces the burden of unfolded proteins.
Targeting Irreversibly Damaged Proteins for Degradation
When chaperones fall short in rescuing proteins, BAG1 directs chaperones to the ubiquitin–proteasome pathway. BAG1 forms complexes with ubiquitin ligases and proteasomal components, which ensures rapid clearance of damaged proteins. This prevents proteotoxic stress, which is a major trigger of cell death in neurodegenerative diseases.
Stabilizing Key Regulators of Survival Pathways
The stability of kinases, transcription factors, and other survival-promoting proteins depends on Hsp70 chaperoning. BAG1 improves chaperone efficiency and indirectly supports growth-promoting signaling cascades.
Isoform Diversity and Functional Specialization
The BAG1 gene produces multiple protein isoforms that differ in size, intracellular localization, and functional specialization.
BAG1L (50 kDa)
BAG1L is the longest isoform that predominantly resides in the nucleus. It contains a nuclear localization signal and interacts with:
- Transcriptional regulators
- Nuclear hormone receptors
- Chromatin-associated proteins
BAG1L enhances survival in the following two ways:
- It influences gene expression
- It modulates nuclear receptor pathways
BAG1M (46 kDa) and BAG1S (33 kDa)
BAG1M (46 kDa) and BAG1S (33 kDa) primarily modulate chaperone-mediated survival and participate in:
- BCL-2 regulation
- Hsp70 cycling
- Proteasomal targeting
BAG1S is rapidly upregulated during acute stress events. It is essential for immediate cytoprotective responses.
p29 BAG1
It is the shortest isoform and lacks a significant portion of the N-terminal region. However, p29 BAG1 retains the BAG domain. Significantly associated with proteasomal function, p29 BAG1 has been detected in both cytosolic and nuclear compartments.
Isoform-specific functions allow cells to coordinate:
- Apoptotic suppression
- Stress response
- Transcriptional regulation
Mouse BAG1 monoclonal antibodies are validated to detect multiple isoforms. Researchers use these antibodies to study differential BAG1 expression patterns across tissues and experimental models.
BAG1 in Tumor Progression and Chemoresistance
BAG1 overexpression has been documented in breast cancer, non-small-cell lung cancer, colorectal carcinoma, glioblastoma, melanoma, and hematologic cancers.
BAG1 contributes to tumorigenesis in the following ways:
Suppression of Apoptosis
It allows cancer cells to survive genomic instability and oncogenic stress.
Enhancement of Hormone Receptor Signaling
It promotes growth in hormone-dependent tumors.
Stabilization of RAF-1 Kinase
It supports MAPK/ERK signaling, which contributes to proliferation and survival.
Support of Proteostasis
It helps cancer cells withstand metabolic and oxidative stress within the tumor.
Protection Against Chemotherapy
High BAG1 levels correlate with resistance to:
- Cisplatin
- Paclitaxel
- Doxorubicin
- Radiation
BAG1 and Neuroprotection
Neuronal apoptosis often leads to irreversible functional loss. BAG1 reduces neurotoxicity and plays a protective role in neurons.
- It facilitates the degradation of misfolded proteins associated with:
- Alzheimer’s disease
- Parkinson’s disease
- Huntington’s disease
- It supports mitochondrial health under oxidative stress.
- It modulates glucocorticoid receptor signaling, influencing neuronal survival.
- It stabilizes critical neuronal proteins.